Why are scientists so excited about the newest malaria vaccine?
Posted on 5/7/21 by Laura Snider
It may be surprising that in a world where we’ve developed vaccines to protect us against everything from polio to the seasonal flu, there isn’t (yet) a highly effective and widely available vaccine against malaria, a disease that infects around 229 million people and kills almost 400,000 people a year. Researchers have been working for decades to try to find a way to prevent this disease, with only limited success.
Recently, though, there’s been some exciting news about malaria vaccines. At the end of April, the results of a clinical trial in Burkina Faso showed that a new candidate malaria vaccine, R21, was more effective than any of its predecessors. Scientists all over the world are cautiously optimistic about this new vaccine—in this blog post, we’re going to look at the reasons why.
What is malaria?
Before we talk about malaria vaccines, let’s go over the basics of malaria itself.
Malaria is a serious, sometimes fatal, disease spread by Anopheles mosquitoes. It is caused by one of four types of parasite: Plasmodium falciparum, P. vivax, P. ovale, or P. malariae. When a female Anopheles mosquito feeds on the blood of an infected person, the parasites enter the mosquito’s gut and reproduce there. As the parasites mature, they travel to the mosquito’s salivary glands, and when that mosquito feeds again, it injects its parasite-infected saliva into the person it bites. The parasites infect the liver, and subsequently the red blood cells, of their human host. Consequently, malaria can be diagnosed by looking at a blood smear under a microscope.
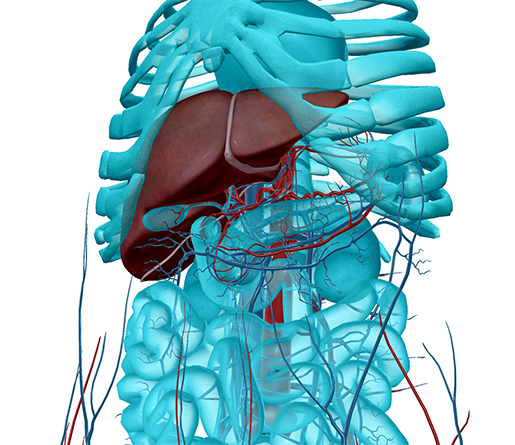 The liver and the blood vessels of the abdomen. Image from Human Anatomy Atlas.
The liver and the blood vessels of the abdomen. Image from Human Anatomy Atlas.
The typical symptoms of malaria are flu-like. They include chills, sweats, fever, headaches, body aches, nausea, and vomiting. Anemia and jaundice may also occur, as a result of the loss of red blood cells. It is important that those with malaria receive treatment quickly—kidney failure, seizures, and even death can result if it goes untreated.
Anopheles mosquitoes and the parasites they carry can be found in many countries with tropical and subtropical climates. According to the CDC, malaria transmission is possible in “large areas of Africa and South Asia and parts of Central and South America, the Caribbean, Southeast Asia, the Middle East, and Oceania.” Travelers to these areas can take antimalarial medication to reduce the risk of contracting malaria during their trip.
Why has developing an effective malaria vaccine been so hard?
Sub-Saharan Africa sees around 90% of deaths from malaria (which total around 400,000 per year worldwide), and most people who die from malaria are children under five years old. An effective vaccine could therefore make a huge difference and save many lives, but despite years of research and numerous clinical trials, it has been difficult to produce a vaccine that is effective enough to make a difference in the fight against malaria. Why?
First of all, the parasites that cause malaria have thousands of genes and a complex life-cycle that involves both sexual and asexual reproduction. Malaria infection in humans begins in the liver, where forms of the parasite called sporozoites reproduce asexually to produce merozoites. Merozoites are the forms of the parasite that go out into the bloodstream and infect red blood cells. Some merozoites will go on to become gametocytes, the sexually-reproducing form of the parasite. Gametocytes get picked up by mosquitoes when they feed on the blood of an infected person.
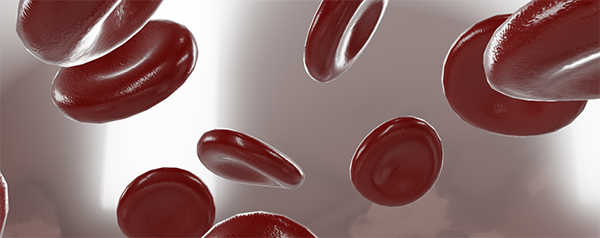 Red blood cells, or erythrocytes, carry oxygen from the lungs to the rest of the body. Many of malaria’s symptoms are the result of infected red blood cells bursting. Image from Visible Body’s 3D Biology models.
Red blood cells, or erythrocytes, carry oxygen from the lungs to the rest of the body. Many of malaria’s symptoms are the result of infected red blood cells bursting. Image from Visible Body’s 3D Biology models.
All of this means that when a vaccine is developed, researchers must decide which stage of the parasite’s life cycle to target. For example, RTS,S/Mosquirix—a malaria vaccine that is currently being implemented in Ghana, Kenya, and Malawi through a program that started in 2019—was designed to prevent sporozoites from infecting the liver. The WHO reports that “among children aged 5–17 months who received 4 doses of RTS,S, the vaccine prevented approximately 4 in 10 (39%) cases of malaria over 4 years of follow-up and about 3 in 10 (29%) cases of severe malaria, with significant reductions also seen in overall hospital admissions as well as in admissions due to malaria or severe anaemia.” Overall, Mosquirix is around 56% effective after one year and 36% effective after three years, making it the most effective malaria vaccine to date.
Malaria also doesn’t work quite like a lot of other diseases people receive vaccinations for (namely, those caused by viruses or bacteria) when it comes to adaptive immunity. People who have already had chickenpox or measles, or the vaccines for them, won’t contract those diseases. In comparison, the immunity conferred by previous malaria infection is only partial. People who have already had malaria can get it again, though it most likely will be less severe after the first time.
What's different about R21?
R21, the new vaccine candidate developed by Oxford University researchers in partnership with Novavax and the Institute de Recherche en Sciences de Santé (IRSS) - Clinical Research Unit of Nanoro, has generated excitement among scientists as it moves into its next phase of trials. Here’s the reason: in a year-long study of 450 5- to 17-month-old children in Burkina Faso, R21 proved to be up to 77% effective in preventing malaria. This exceeds the WHO’s goal of 75% efficacy for a malaria vaccine, and would make it more effective than Mosquirix.
In fact, R21 is a modified version of Mosquirix. It seeks to elicit an antibody response by introducing a protein that malaria parasites make in their sporozoite phase. The concentration of these proteins is higher in R21, and it also includes an adjuvant (an immune-boosting compound) that is easier to produce than the one in Mosquirix.
The results of larger-scale testing of R21—which will include a larger test group of 4,800 children in Burkina Faso, Mali, Kenya, and Tanzania—remain to be seen, but its success so far is promising. Hopefully, R21 means that the world is one step closer to having an effective malaria vaccine that can easily be mass produced and distributed to those who need it.
Be sure to subscribe to the Visible Body Blog for more anatomy awesomeness!
Are you an instructor? We have award-winning 3D products and resources for your anatomy and physiology course! Learn more here.