Free Lesson Plan: Diffusion and Osmosis with Visible Body Suite
Posted on 1/21/22 by Sarah Boudreau
Diffusion and osmosis are concepts that are important in understanding how cells—and organisms at large—function. Rather than let students “learn through osmosis,” we here at Visible Body have put together a lesson plan that uses visuals to illustrate how diffusion works.
By the end of this lesson, students will learn…
- The factors that influence the rate of diffusion
- The difference between diffusion and osmosis
- How a selectively permeable membrane works
- The following vocabulary:
- Active transport
- Passive transport
- Diffusion
- Osmosis
- Concentration gradient
- Selectively permeable membrane
- Isotonic, hypotonic, and hypertonic
- Osmotic pressure
In addition to VB Suite access, this lesson will require the following:
- Iodine
- Starch
- A Ziploc bag
- Three beakers or glasses
- Food coloring
- Hot and cold water
Step 1: Overview
At the beginning of the lesson, prepare the iodine example for step 3 in front of the students:
- Place a teaspoon of starch into the plastic bag.
- Fill a glass halfway with water and add ten drops of iodine.
- Place the bag of starch into the solution.
At this point in the course, students are aware of the parts of the cell and the basics of what cells need to function. To begin this lesson, we’ll take what they know (parts of the cell) and use it as an entry point to understand diffusion.
Introduce the idea that there are two ways for molecules to move into the cell: active transport and passive transport (plus phagocytosis and pinocytosis).
Active transport uses energy, passive transport does not. We will focus on passive transport today, examining diffusion and a type of diffusion called osmosis.
Introduce the term concentration gradient and explain how this concept is central to understanding diffusion.
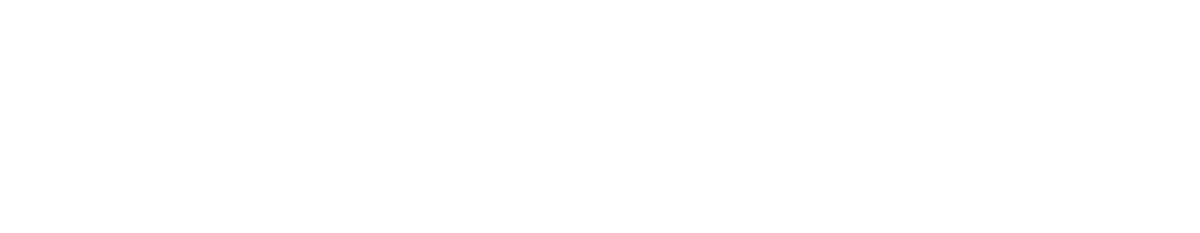
Start by illustrating a gradient using colors. Pull up one of the cell models in VB Suite. Next to the cell, use the drawing tool to draw a color gradient as illustrated in the image below. The transparency tools will help you transition from saturated color to blank space. Point out to the students how one side is more weighed down with color than the other, making things uneven.
 The concentration gradient represented by a color gradient.
The concentration gradient represented by a color gradient.
Using the same color and a semitransparent brush, draw a rectangular block of diluted color to represent equilibrium. Compare the color gradient to the equalized block of color.
Next, explain how this same idea applies to molecules on either side of a cell membrane. Particles in a fluid move around randomly; this is known as Brownian motion. The higher the concentration of particles, the more likely molecules are to bump into other molecules and gain enough energy to bounce away. In lower concentrations, that happens less often. Diffusion happens when particles move “down” a concentration gradient from an area of higher concentration to an area of lower concentration. When there is no more gradient, there is no more energy difference between areas and so movement in all directions becomes equal.
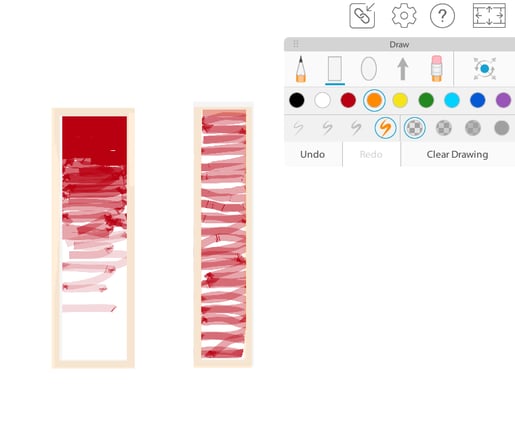
Using the drawing tool again, draw a sequence of oxygen molecules in a gradient moving into the cell.
A concentration gradient using the drawing tools and animal cell model in VB Suite.
If you're looking for more resources, this video from our Visible Biology YouTube series provides a fun, easy-to-understand overview of diffusion:
Lesson on diffusion from the Visible Biology YouTube series with Dr. Cindy Harley.
Review questions for students:
- Describe the difference(s) between active and passive transport.
- What is a concentration gradient?
Step 2: Diffusion Rates
Next, show the students diffusion in action and explore the factors that influence the rate at which diffusion takes place.
Take two beakers and pour hot water in one and cold water in the other. Drop 3-4 droplets of food coloring into each of the beakers and ask students to note how quickly the food coloring diffuses in the different beakers.
Ask the students why the hot water beaker and the cold water beaker might have different diffusion rates.
Next, walk the students through the factors that influence diffusion rates. This table is adapted from Biology LibreText, an open-source textbook:
|
Extent of the concentration gradient |
The greater the difference in concentration, the more rapid the diffusion. The closer the distribution of the material gets to equilibrium, the slower the rate of diffusion becomes. |
|
Mass of the molecules diffusing |
Heavier molecules move more slowly; therefore, they diffuse more slowly. The reverse is true for lighter molecules. |
|
Temperature |
Higher temperatures increase the energy and therefore the movement of the molecules, increasing the rate of diffusion. Lower temperatures decrease the energy of the molecules, thus decreasing the rate of diffusion. |
|
Solvent density |
As the density of a solvent increases, the rate of diffusion decreases. The molecules slow down because they have a more difficult time getting through the denser medium. If the medium is less dense, diffusion increases. Because cells primarily use diffusion to move materials within the cytoplasm, any increase in the cytoplasm’s density will inhibit the movement of the materials. An example of this is a person experiencing dehydration. As the body’s cells lose water, the cytoplasm becomes denser, and the rate of diffusion decreases in the cytoplasm, and the cells’ functions deteriorate. Neurons tend to be very sensitive to this effect. Dehydration frequently leads to unconsciousness and possibly coma because of the decrease in diffusion rate within the cells. |
|
Solubility |
As discussed earlier, nonpolar or lipid-soluble materials pass through plasma membranes more easily than polar materials, allowing a faster rate of diffusion. |
|
Surface area and thickness of the plasma membrane |
Increased surface area increases the rate of diffusion, whereas a thicker membrane reduces it. |
|
Distance traveled |
The greater the distance that a substance must travel, the longer it takes for molecules to get there. This places an upper limitation on cell size. A large, spherical cell will die because nutrients or waste cannot reach or leave the center of the cell. Therefore, cells must either be small in size, as in the case of many prokaryotes, or be flattened, as with many single-celled eukaryotes and animals with no circulatory system (flatworms, for example). |
Note that concentration gradients can act in opposite directions. For example, Na+ may move in one direction and K+ in the opposite direction. Unless they are charged, crowded, etc., diffusing molecules are not affected by other concentration gradients.
Review questions for students:
- Why did the beakers of hot and cold water have different diffusion rates?
- What other factors influence diffusion?
Step 3: Osmosis and Selectively Permeable Membranes
Unlike the food coloring example in step 2, where the food coloring and the water have no barrier between them, cells have membranes that hold organelles in and keep other things out. Describe how when diffusion of water occurs across a membrane, it’s known as osmosis.
To illustrate osmosis, return to the starch and iodine example you prepared earlier. Explain that iodine changes its color when it comes into contact with starch. By now, the starch in the baggie will have turned purple because the plastic bag is permeable to iodine; the iodine has moved across the “membrane.”
In a cell, molecules need to move in and out of the cell, but how does the cell membrane let only certain molecules pass through?
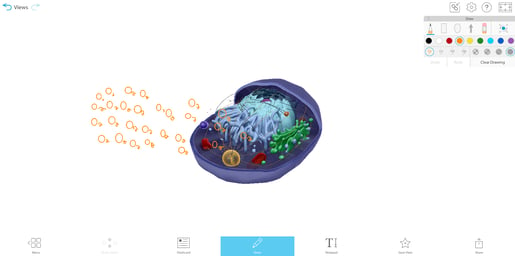
To help students understand selective permeability, watch the cell transport animation in VB Suite. Discuss how two sheets of phospholipids create a selectively permeable membrane that lets only small molecules through.
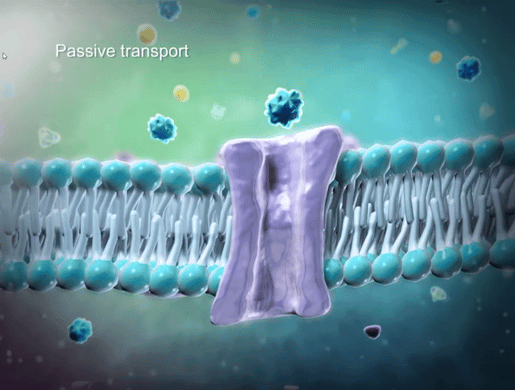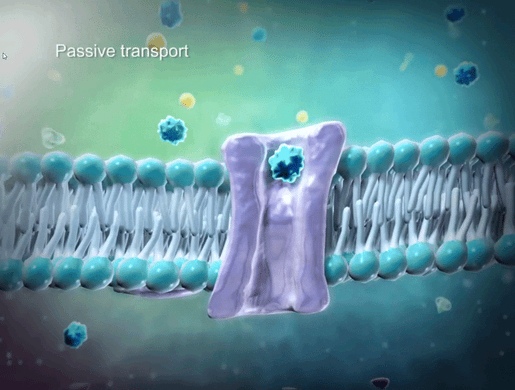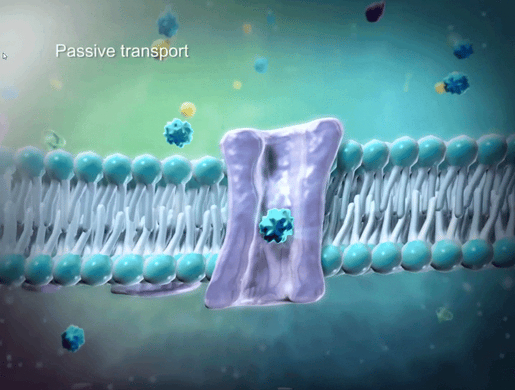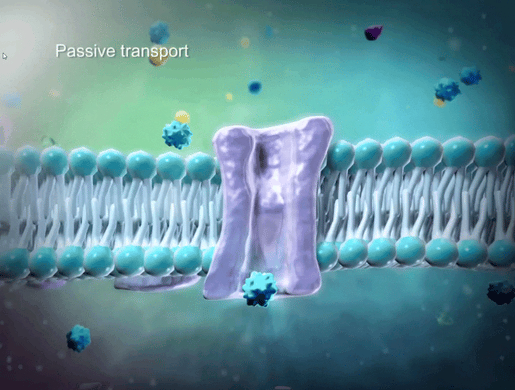 GIF from VB Suite.
GIF from VB Suite.
Return to the VB Suite cell model and the drawing tool. Using the drawing tool, draw many other molecules outside of the cell and a few on the inside and use arrows to illustrate the movement of molecules from one side of the concentration gradient to the other.
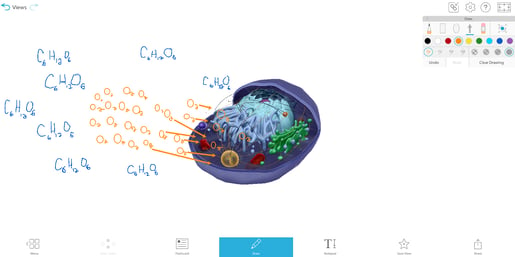 Oxygen molecules move through osmosis into the cell while glucose molecules remain outside.
Oxygen molecules move through osmosis into the cell while glucose molecules remain outside.
Introduce the concept of osmotic pressure.
Next, introduce the different terms that describe relationships between sides of the membrane. The definitions below are from Biology Online.
|
Isotonic |
Solutions that are categorized as having equivalent or identical osmotic pressure |
|
Hypotonic |
Having a lesser osmotic pressure in a fluid compared to another fluid |
|
Hypertonic |
Having a greater osmotic pressure in a fluid compared to another fluid |
Review questions for students:
- What is a selectively permeable membrane?
- What is the difference between the terms hypotonic, hypertonic, and isotonic?
Step 4: The Big Picture
Finally, briefly connect osmosis and diffusion to the big picture: how does diffusion affect other processes?
Here are some ideas for “big picture” connections:
- During cellular respiration, diffusion occurs when oxygen moves into the cell and when carbon dioxide moves out.
- Oxygen moves into the lungs via diffusion.
- Check out this webinar on comparative physiology of the heart by Dr. Cindy Harley that discusses diffusion and hearts.
NGSS Standards
The above lesson plan fits these Next Generation Science Standards (NGSS):
HS-LS1 From Molecules to Organisms: Structures and Processes. Students who demonstrate understanding can:
- Develop and use a model to illustrate the hierarchical organization of interacting systems that provide specific functions within multicellular organisms. (HS-LS1-2)
Did you know that the Visible Body Education Team has created a library of lesson plans and lab activities? Here's where you can find these resources, complete with NGSS standards.
Be sure to subscribe to the Visible Body Blog for more anatomy awesomeness!
Are you an instructor? We have award-winning 3D products and resources for your anatomy and physiology course! Learn more here.